My bag of extra clothes was a constant reminder of heavy bleeding.
With lighter periods, it’s another thing off my shoulders.

Myfembree makes lighter periods possible
Myfembree was studied and proven effective in two 6-month clinical studies involving a total of 768 premenopausal women who had heavy period bleeding due to uterine fibroids.
After taking Myfembree, average
period bleeding went down by
Myfembree is not a surgery, procedure, injection, or painkiller. It is the first and only FDA-approved once-daily pill to reduce heavy menstrual bleeding from uterine fibroids in premenopausal women.
women saw a meaningful decrease in period bleeding†‡
†Normal bleeding is defined as 80 mL (about 1/3 cup) or less.
‡At week 24, compared with 16% of women on placebo.

What could you expect when taking Myfembree?
Your next period could be lighter. Within 1 cycle, some women had less period bleeding.
While the studies were not specifically designed to determine how quickly Myfembree worked, some of the women started to see a reduction in bleeding at Week 4.
Half of women stopped getting their periods during the last month of treatment in the studies.§ A majority of women saw their periods return once they stopped the therapy,‖ typically within 1-2 months.
§Compared with 5% of women on placebo.
‖Based on 65 women who did not enter a post-treatment extension study or stopped treatment earlier.
What are uterine fibroids?
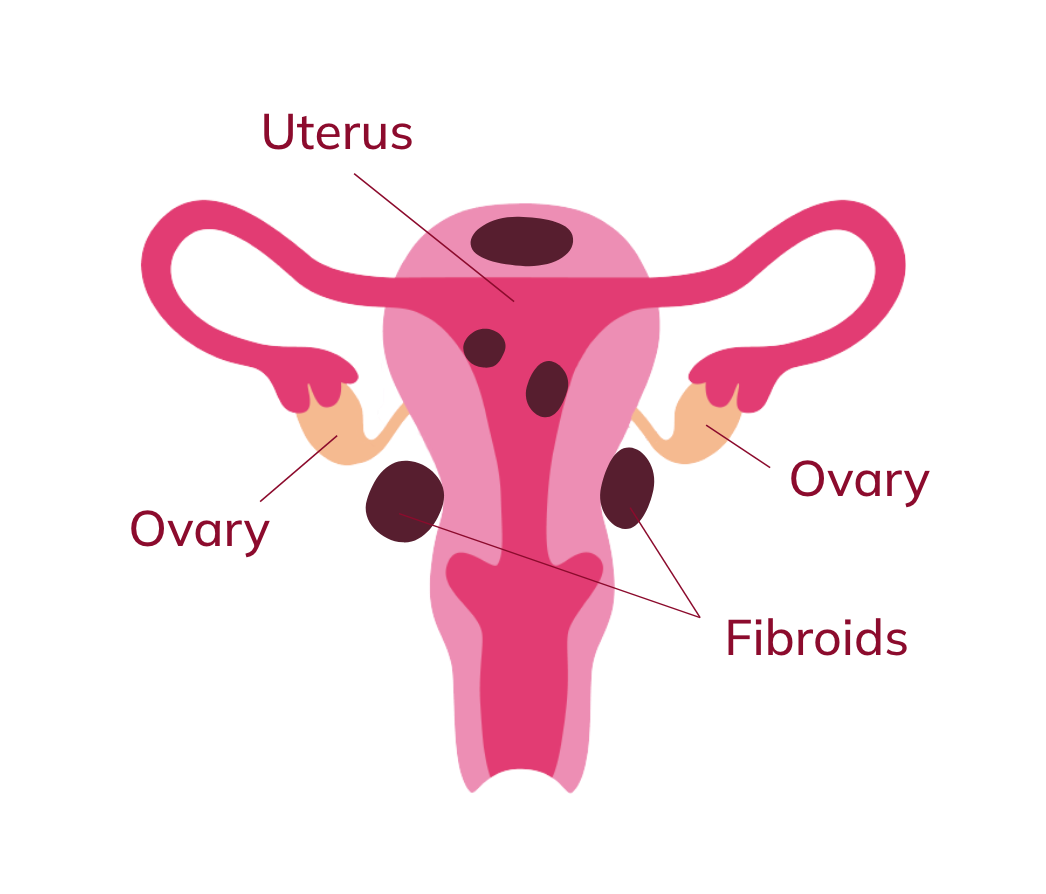
Are your periods feeling heavy? Uterine fibroids (also called uterine myoma) are a common cause. While it’s not known exactly what causes uterine fibroids, they are non-cancerous and develop as growths that are found in and around the uterus. The main symptom of UF is heavy menstrual bleeding.
Symptoms from fibroids may make dealing with periods challenging, such as bleeding for more than 7 days, requiring more than one pad/tampon at a time, and feeling dizzy or fatigued due to anemia from UF-related heavy menstrual bleeding.
How does Myfembree work to reduce bleeding from uterine fibroids?
Myfembree contains three key ingredients, designed to support an optimal hormone range that may help reduce heavy bleeding due to fibroids.
Relugolix:
Reduces hormones, such as
estrogen, to reduce heavy bleeding.Norethindrone acetate:
May protect the uterus from the effect of estrogen alone.
Estradiol (an estrogen):
May reduce potential bone loss from relugolix alone.
What are the possible side effects with Myfembree?
In clinical trials, the safety of Myfembree was also studied.

The most common side effects
The most common side effects of taking Myfembree for heavy period bleeding due to UF include hot flushes, increased sweating, night sweats, abnormal vaginal bleeding (bleeding that lasts too long, is too heavy, or is unexpected), hair loss or hair thinning, and decreased interest in sex. Always tell your doctor if you experience a side effect that bothers you or will not go away.

Serious side effects
Serious side effects were reported in 3.1% of women on Myfembree vs 2.3% on placebo. Fibroid expulsion with heavy bleeding, fibroid prolapse, gallbladder inflammation, and pelvic pain were experienced by one person each across both studies.

Discontinuations
In clinical trials, 3.9% of women treated with Myfembree stopped taking Myfembree as a result of side effects, compared to 4.3% of women in the placebo group. The most common side effect that led to discontinuation was uterine bleeding (1.2%), occurring usually within the first 3 months of treatment.